Ginkgo.Your Innovation Partner.
We were incredibly lucky to have Andrea Ling spend 3 months at Ginkgo Bioworks as our 2019 Creative Resident, exploring how to design a world without waste. Here she reflects on her residency, sharing her work and learnings.
[caption id="attachment_3879" align="aligncenter" width="1866"]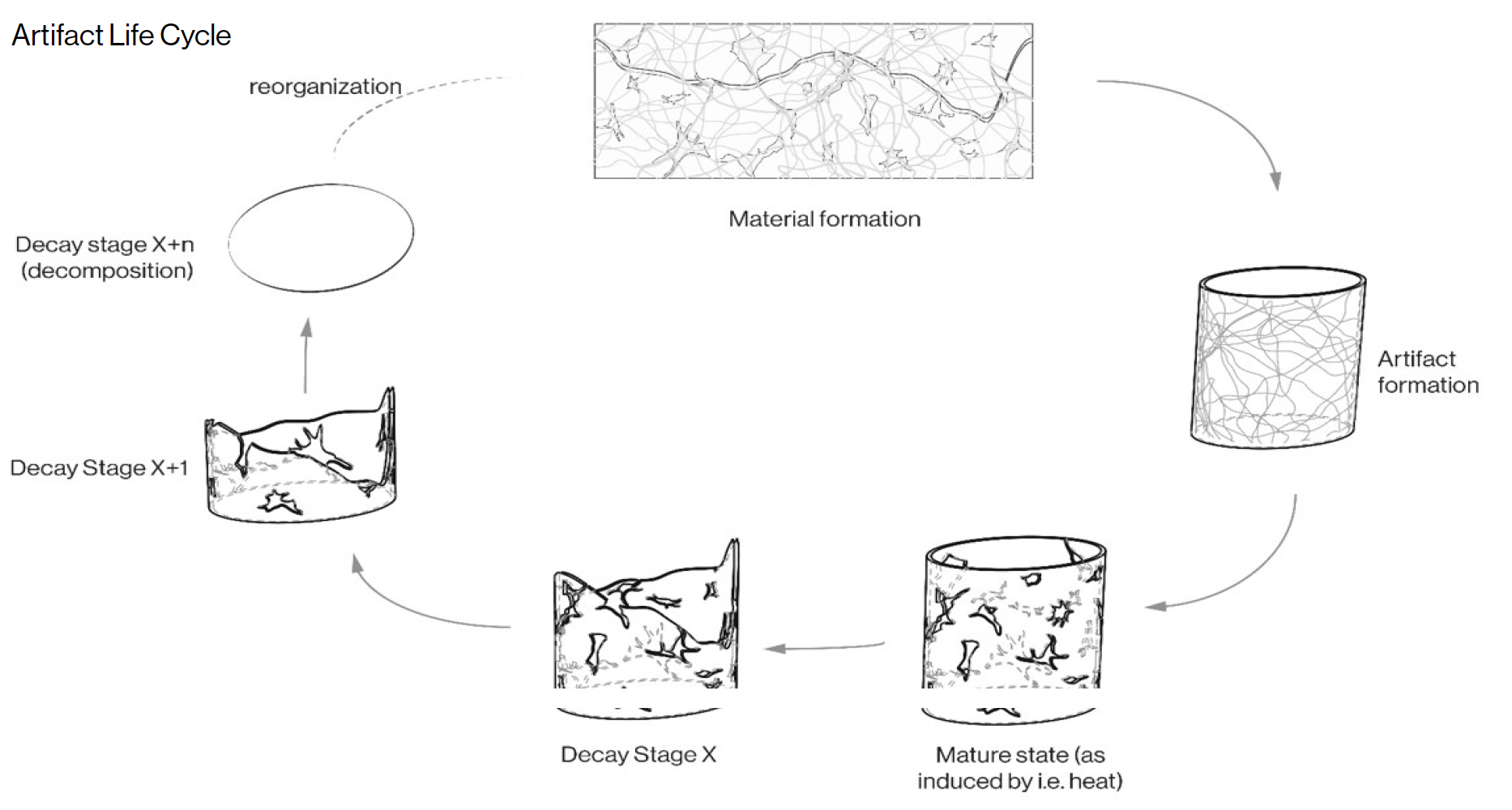 Image 1: Artifact Life Cycle diagramming formal transformations of material to artifact and back to material with possible transformation mechanisms indicated.[/caption]
Image 1: Artifact Life Cycle diagramming formal transformations of material to artifact and back to material with possible transformation mechanisms indicated.[/caption]
The main material system I worked with during my residency was a set of biologically derived and biodegradable polymers I was introduced to as a research assistant at the MIT Media Lab. Chitosan (a structural polysaccharide found in insect and crustacean shells and fungal walls), cellulose (a structural polysaccharide found in plant walls), and pectin (a polysaccharide found in fruit skins) are some of the most abundant biopolymers on the planet, and are water soluble with short decay cycles. They can be harvested from waste from shrimp farms (chitosan), wood pulp mills (cellulose) and agriculture waste (pectin). When made into water-based colloids, they can be extruded into forms or cast into sheets of bioplastic that are dried and can then be further processed into forms or used as fodder for microbes to take up. And while the gradation and proportions of these ingredients can be tuned to create a vast design space of characteristics including opacity, colour, flexibility, and mechanical strength, the use of a minimal inventory of components – in this case, 3 structural polysaccharides that are simply processed – ensures that the entire library can accommodate biodegradation with ease. That is, a whole artifact is made of a small set of ingredients which do not require vastly different end-of-life processes to decompose and can be easily taken up again into a nutrient cycle. This is in contrast to many industrial material systems, where assemblies of things must be separated into the constituent parts and taken to different recycling facilities, scrap yards, and landfills for disposal.
[caption id="attachment_3860" align="aligncenter" width="2560"] Image 2: In Bioworks 1, at the Streptomyces work stations. Photo by Ally Schmaling.[/caption]
Image 2: In Bioworks 1, at the Streptomyces work stations. Photo by Ally Schmaling.[/caption]
Much of my experience with biological materials came from working at the Media Lab on the Aguahoja project, a collection of artifacts and a pavilion made of these biodegradable composites. I am immensely proud of what my teammates and I were able to achieve together and was heartbroken to learn that our project, a model for regenerative consumption and restored balance with nature, was in part facilitated by money and power dynamics that fed on the assault of young women.
Why is this relevant background for my experience during Ginkgo’s creative residency, a decidedly optimistic one on designing a world with the assistance of biological technologies? Because the extractive capitalist system that can traffic girls as bodies to be exploited is the same one that currently views biology as the next great resource to be mined. I was struck by this when I read Jason Kelly’s shareholder letter in GROW, Ginkgo’s new publication. And while I in no way mean to imply that Ginkgo’s work is in any way related to the problematic funding issues at MIT, Ginkgo has to survive the same extractive economy. And so, in his letter, Jason describes how in the 1930s, IBM CEO Thomas Watson, when explaining what computers could do with data to a journalist, relied on terms borrowed from industrial iron ore extraction to explain how data could be mined and then processed into different forms similar to how other raw materials could be transformed into finished goods that are consumed. Watson was considered one of the greatest salesmen of his time, and his metaphor, the foundation for how we still speak of computing today, provided consumers a way of understanding data processing in terms of extractive production and ensuing economic efficiency.
Synthetic biology in turn relies heavily on computing metaphors to explain the ability to program organisms into tools of our making. We use this language because it makes the programming of life easier to describe (and easier for investors to understand) implying the level of control we have when we program digital code. Synthetic biology companies inherit and promote the benefits of these programmatic and economic implications in order to expand their business. But this language is limited in its ability to communicate the restorative power of biology, instead relying on terms based on resource depletion, dominance, and endless consumption as well as assumption about how controllable life can be. Where in a “healthy” extractive capitalist system, unceasing consumption is required for ever increasing economic growth, a biological system has limits. Biology’s currency is energy and matter, of which there is a finite amount. Its efficiency is metabolic, not economic and not lean, and based on redundancy, leakiness, and transience. It cannot be controlled the same way we can control industrial mechanisms. And it cannot be raped without any provision of return, for without nurture and restoration, the system will collapse. But if tended to, in return, biological systems can provide a far more robust system of growth, renewal, and system longevity that extractive systems are not capable of.
[caption id="attachment_3885" align="aligncenter" width="2014"] Image 3: Left side, biological material system experiments using pectin-chitosan-cellulose composites. Right side, tripod structures made of pectin-chitosan-cellulose composites using enzymatic degradation (pectinases and chitonases) as subtractive fabrication tool. Photos by Ally Schmaling.[/caption]
Image 3: Left side, biological material system experiments using pectin-chitosan-cellulose composites. Right side, tripod structures made of pectin-chitosan-cellulose composites using enzymatic degradation (pectinases and chitonases) as subtractive fabrication tool. Photos by Ally Schmaling.[/caption]
Jason closes his shareholder’s letter along these lines. He writes: “In nature, growth is almost never exponential, but embedded in complex relationships that guide and shape it. Growth requires nurturing. We cannot create growth, we can only create the best conditions for something to grow...we must recognize that there are no externalities, no “outside” when we are growing technologies with living things.” For Ginkgo, a great opportunity of the creative residency is to provide new visions, models, and metaphors, to shape a new way of designing not through the imperatives of capital but through the power of biology.
It is in this context that I would like to situate my interest in designing decay and my time at Ginkgo. In decay, we can return material and energy back to the system and by designing it, we can perhaps bias how decay might happen so that we can live with the consequences in sight and on site. By mediating the decay process through species selection, control of environmental conditions, and templating of nutrients, I am actively pursuing self-renewing forms and mutability as a desired quality in the physical world as well as guarantee that the mechanisms of constructive renewal will be embedded into that world.
[caption id="attachment_3855" align="aligncenter" width="1707"] Image 4: In Bioworks 1, in the spore room, plating Aspergillus niger. Photo by Grace Chuang.[/caption]
Image 4: In Bioworks 1, in the spore room, plating Aspergillus niger. Photo by Grace Chuang.[/caption]
I have always been a process-focused designer, in that I spend more time designing how to make something than I do designing what to make, and my time at Ginkgo was no different. The first thing I did at Ginkgo was run a short biomaterials workshop with interested Ginkgo engineers and scientists where I shared my experience working with these materials as compared to traditional manufacturing techniques used to form petroleum-based plastics. The workshop also afforded me the opportunity to charette with Ginkgo staff how they would work with these materials and strategies to facilitate decay. Afterwards, I worked on 3 parallel streams of research, all revolving around understanding decay as a fabrication process. How could we harness the responsivity and temporality of biologically derived materials in order to shape new material? The work partitioned itself into 3 smaller projects:
Using enzymatic degradation of bioplastics as both a means of transforming material instead of destroying material. These tests were done with the mentorship of Joshua Dunn, head of design at Ginkgo, and with advice from Nikos Reppas, foundry director.
Using different species of Streptomyces bacteria to colonize cellulose and bioplastic substrates in order to transform them. The Streptomyces genus encompass a group of common soil bacteria that are capable of facilitating biodegradation but are not considered robust decay agents. They leave evidence of their metabolic activity with the release of vibrant pigments, transforming the materials they colonize. These tests were done with the guidance of Kyle Kenyon and with advice from Duy Nguyen and Lucy Foulston, all Streptomyces researchers.
Using different types of fungi, including Aspergillus niger (black mold) and Trichoderma viride (green mold) in co-cultures to transform and degrade different materials. Molds are much more powerful and resilient decay agents compared to Streptomyces and would rapidly colonize any substrate we provided. These tests were done with the expertise of Ming-Yueh Wu, one of Ginkgo’s fungi engineers.
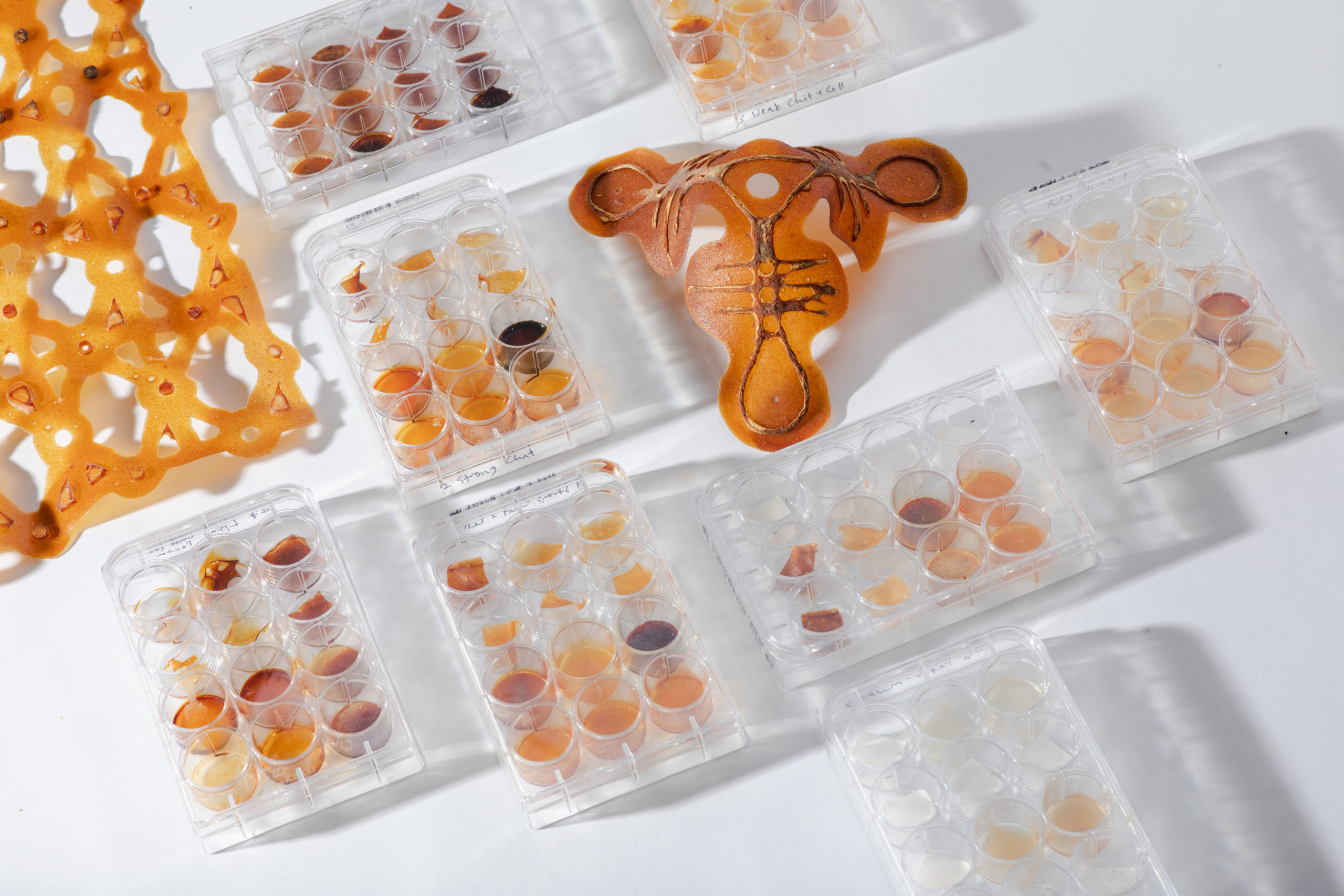 Image 5: Enzymatic degradation tests charting effects of different dilutions of pectinase, chitanse, cellulase, amylase, and lysing enzymes on material system. Photo by Ally Schmaling.[/caption]
Image 5: Enzymatic degradation tests charting effects of different dilutions of pectinase, chitanse, cellulase, amylase, and lysing enzymes on material system. Photo by Ally Schmaling.[/caption][caption id="attachment_3880" align="aligncenter" width="1707"] Image 6: Cocoon for bio-transformation made of laser cut chitosan-cellulose composite. Photo by Ally Schmaling.[/caption]
Image 6: Cocoon for bio-transformation made of laser cut chitosan-cellulose composite. Photo by Ally Schmaling.[/caption]
[caption id="attachment_3870" align="aligncenter" width="1707"] Image 7: Large final samples including contaminated wood block originally inoculated with Streptomyces coelicolor, pectin-chitosan-cellulose ‘painting’, and cocoon sample made of chitosan film. Photo by Ally Schmaling.[/caption]
Image 7: Large final samples including contaminated wood block originally inoculated with Streptomyces coelicolor, pectin-chitosan-cellulose ‘painting’, and cocoon sample made of chitosan film. Photo by Ally Schmaling.[/caption]
[caption id="attachment_3844" align="aligncenter" width="1707"] Image 8: Initial tests culturing different forms of cellulose and chitosan-cellulose composites with Streptomyces coelicolor. Photo by Ally Schmaling.[/caption]
Image 8: Initial tests culturing different forms of cellulose and chitosan-cellulose composites with Streptomyces coelicolor. Photo by Ally Schmaling.[/caption]
[caption id="attachment_3849" align="aligncenter" width="1707"] Image 9: Wood blocks inoculated with Aspergillus niger and Trichoderma viride, as well as 2 contaminated wood blocks (species unknown). Photo by Ally Schmaling.[/caption]
Image 9: Wood blocks inoculated with Aspergillus niger and Trichoderma viride, as well as 2 contaminated wood blocks (species unknown). Photo by Ally Schmaling.[/caption]
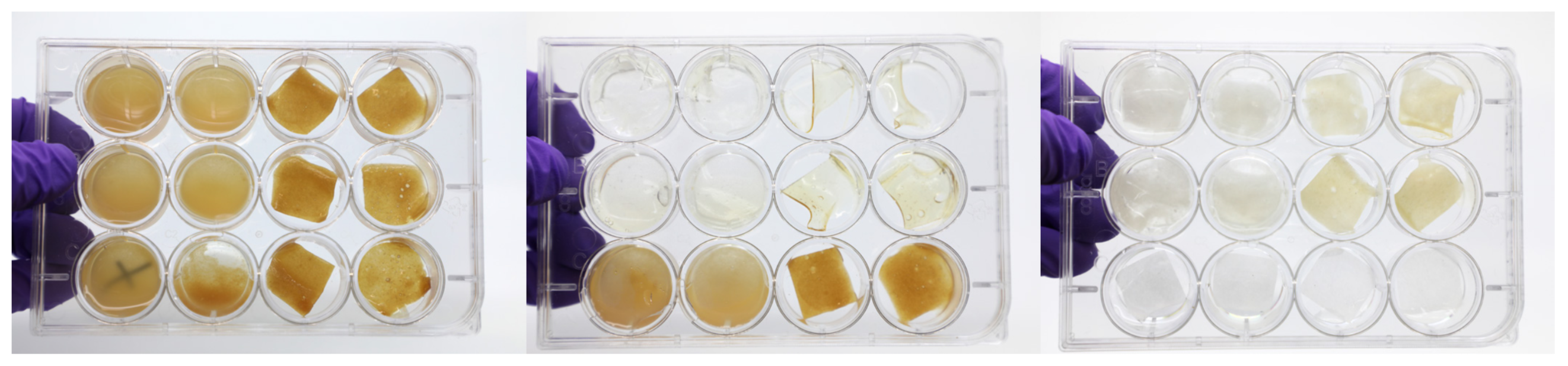 Image 10: Enzymatic degradation tests charting effects of different dilutions of pectinase, chitanse, cellulase, amylase, and lysing enzymes on pectin-chitosan, chitosan, chitosan-cellulose, and cellulose samples. Photos by Andrea Ling.[/caption]
Image 10: Enzymatic degradation tests charting effects of different dilutions of pectinase, chitanse, cellulase, amylase, and lysing enzymes on pectin-chitosan, chitosan, chitosan-cellulose, and cellulose samples. Photos by Andrea Ling.[/caption]Josh and I tested out a series of different enzymes—pectinase, cellulase, chitinase, amylase, and a lysing enzyme cocktail—at different dilutions to test their effect on my composite materials. Enzymatic degradation was tested both through spot testing (applying small drops of enzymes to wet and dry material) as well as by submerging the composites in buffer solutions that contained small amounts of enzymes. While the submerging method was the most effective at breaking down material, this also meant that the material was completely soaked and without form during the degradation process. I was very much interested in using the enzymes as fabrication tools and to use enzymes in situ on the artifacts. While the spot tests on dry material were not effective unless the materials were then incubated in extremely high humidity, spot tests on wet and semi-wet material were promising. I was able to use the enzymes to degrade pectin, chitosan, and cellulose skins during their casting process, thus shaping the artifacts using selective degradation in certain areas. That is, I was able to create holes and lines in areas where I applied enzyme. As a control, I made a large pectin-cellulose-chitosan ‘painting’ that I laser cut and dehydrated, comparing its resolution with a similar painting where enzyme was used in lieu of laser cut lines. The enzyme degraded lines were of poor resolution, a function of the enzyme’s diffusion through the semi-wet material and often ate away more material than expected. The remnant material at the lines was a sticky powder and made the intact portion of the bioplastic very difficult to remove from the substrate. Interestingly, degradation continued even when the colloids were dry to the touch, seen as the lines and holes became larger with time. The most compelling results were obtained when using the enzymes to create holes and perimeter lines for a series of tripod structures. Here, the material was successfully transformed through degradation into objects though not identical, where similar enough that I could use them as consistent tiles for a larger aggregate structure.
[caption id="attachment_3886" align="aligncenter" width="2722"] Image 11: Pectin-chitosan-cellulose ‘paintings’ using 2 different degradation methods. Left image shows painting 1 before using a laser cutter and dehydration to subtract material from the object. Right image shows painting 2 using enzymatic degradation to subtract material from wet colloid. Photos by Andrea Ling.[/caption]
Image 11: Pectin-chitosan-cellulose ‘paintings’ using 2 different degradation methods. Left image shows painting 1 before using a laser cutter and dehydration to subtract material from the object. Right image shows painting 2 using enzymatic degradation to subtract material from wet colloid. Photos by Andrea Ling.[/caption]
[caption id="attachment_3882" align="aligncenter" width="1707"] Image 12: Pectin-chitosan-cellulose ‘paintings’ using 2 different degradation methods. Top right shows painting 1 after using a laser cutter and dehydration to subtract material from the object. Bottom left shows painting 2 after using enzymatic degradation to subtract material from the object. Photo by Ally Schmaling.[/caption]
Image 12: Pectin-chitosan-cellulose ‘paintings’ using 2 different degradation methods. Top right shows painting 1 after using a laser cutter and dehydration to subtract material from the object. Bottom left shows painting 2 after using enzymatic degradation to subtract material from the object. Photo by Ally Schmaling.[/caption]
[caption id="attachment_3841" align="aligncenter" width="2560"]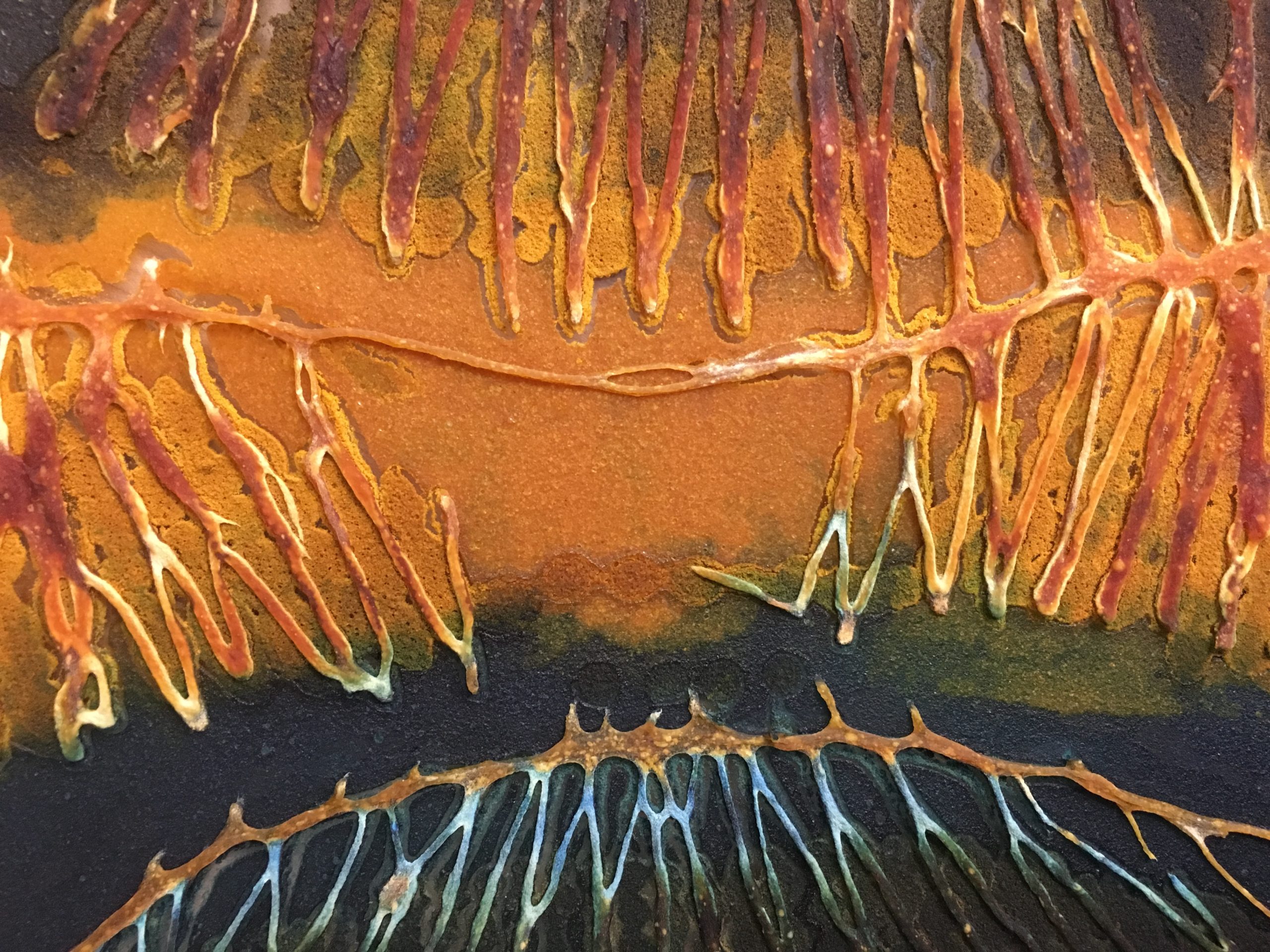 Image 13: Close up of pectin-chitosan film enzyme induced degradation. Photo by Andrea Ling.[/caption]
Image 13: Close up of pectin-chitosan film enzyme induced degradation. Photo by Andrea Ling.[/caption]
[caption id="attachment_3887" align="aligncenter" width="2084"] Image 14: Formed tripod structure using enzymatic degradation as subtractive fabrication tool. Photos by Andrea Ling.[/caption]
Image 14: Formed tripod structure using enzymatic degradation as subtractive fabrication tool. Photos by Andrea Ling.[/caption]
Aspergillus niger is a common black mold found in soil and on fruit and vegetables. It produces pectinase and amylase. Trichoderma viride is a common green mold found in soil that produces cellulases and chitinases and can be used as a bio-fungicide against other plant pathogenic fungi. Combined they smell like humid air. Fungi are extremely effective decomposers, releasing enzymes into decaying material and then absorbing the resulting nutrients. Because they produce airborne spores, the molds have to be cultured in a separate room in Ginkgo so not to contaminate other experiments. Ming helped me test both species on the bio-composites and both were able to grow on the material, provided there was enough moisture. I tried to template their growth using acrylic templates into which I cast nutrient agar to start the fungal cultures and then press a thin sheet of biopolymer onto the template. The fungus would then grow in the pattern onto the bioplastic for a time, eventually eating holes in the material; in one sample it began to over-run the pattern, in another it lost moisture and stayed confined to the original locations. I also tried co-culturing the fungi over the span of a month onto sterile large wooden blocks that had been milled to increase the surface area for colonization. While the Aspergillus colonized the block within a week, the Trichoderma needed about 3 weeks before showing visible green areas. The growth pattern was interesting in that although the Aspergillus and the Trichoderma were plated into different valleys of the wood block, the growth showed them mixing together throughout the block, with the Trichoderma also taking over the flat surfaces. While one of the blocks showed evidence of a third species of mold on it (white mold), both the Aspergillus and Trichoderma were robust enough to withstand the contamination and continue to flourish.
[caption id="attachment_3889" align="aligncenter" width="1138"] Image 15: Sterile maple blocks that have been CNC milled into texture that expands surface area are inoculated with Aspergillus niger and Trichoderma viride and incubated at room temperature for 5 weeks. Photos by Grace Chuang.[/caption]
Image 15: Sterile maple blocks that have been CNC milled into texture that expands surface area are inoculated with Aspergillus niger and Trichoderma viride and incubated at room temperature for 5 weeks. Photos by Grace Chuang.[/caption]
[caption id="attachment_3890" align="aligncenter" width="2284"]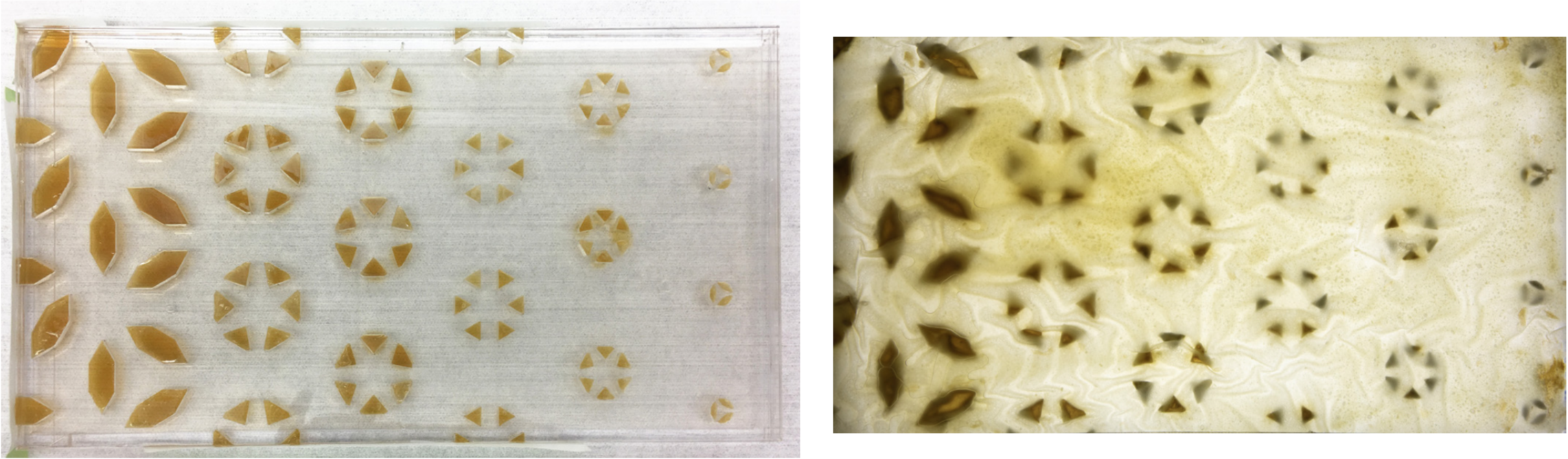 Image 16: Acrylic templates are inoculated with Aspergillus niger and then a chitosan-cellulose film is sandwiched into the template as a subtractive fabrication mechanism. Photos by Andrea Ling.[/caption]
Image 16: Acrylic templates are inoculated with Aspergillus niger and then a chitosan-cellulose film is sandwiched into the template as a subtractive fabrication mechanism. Photos by Andrea Ling.[/caption]
[caption id="attachment_3888" align="aligncenter" width="980"] Image 17: Close ups of wood blocks after 5 weeks incubation; 2 are heavily contaminated with unknown species, 2 have been successfully colonized with Aspergillus niger and Trichoderma viride. Photos by Ally Schmaling.[/caption]
Image 17: Close ups of wood blocks after 5 weeks incubation; 2 are heavily contaminated with unknown species, 2 have been successfully colonized with Aspergillus niger and Trichoderma viride. Photos by Ally Schmaling.[/caption]
 Images 18 & 19: Streptomyces coelicolor cultures on rice paper, inoculated with words “Hello Ginkgo” and “Hello World”. Streptomyces coelicolor cultures on 3 different species of wood (1.6mm thick samples) and chitosan-cellulose film. Photos by Andrea Ling.[/caption]
Images 18 & 19: Streptomyces coelicolor cultures on rice paper, inoculated with words “Hello Ginkgo” and “Hello World”. Streptomyces coelicolor cultures on 3 different species of wood (1.6mm thick samples) and chitosan-cellulose film. Photos by Andrea Ling.[/caption]Streptomyces are a genus of filamentous bacteria that includes over 500 species characterized by a fungal like mycelium body and the production of spores for reproduction. Found predominantly in soil and decaying vegetation, they are a major source of antibiotics (which is why Ginkgo studies them) and produce geosmin, a metabolite that gives soil its characteristic earthy rain smell; many species in the genus also produce vivid pigments as metabolites. Natsai Audrey Chieza, Ginkgo’s first creative resident used Streptomyces coelicolor to naturally dye silk, by culturing the bacteria directly onto the textile in different ways to produce different patterns. Ginkgo provided me with different Streptomyces cultures to test on my bioplastics and see if they could be cultured to dye the materials, transforming them as they also decomposed them. Kyle taught me how to successfully culture S. coelicolor in sterile conditions onto plain cellulose (rice paper) and thin wood substrates however it was exceptionally challenging to get any streptomyces growth onto the composite bioplastics. While we knew that the bacteria were capable of producing cellulases and chitinases and feed on these materials, sterilizing the composites without damaging them or producing toxic residue was an issue. Specifically, autoclaving my dried materials burnt most of them, making them inhospitable to the bacteria, while trying to filter sterilize the viscous wet colloids was not feasible. UV sterilization only partially effective given the opacity of the material. Gas sterilization was not available at Ginkgo. I made a cocoon structure out of my chitosan and cellulose colloids and planned to culture it with the bacteria, recognizing that the perimeter edges of the cocoon strips were burnt from the laser cutter and therefore unlikely to be colonized, but that the interior material was still viable. The initial strip tests, incubated over 30 days, resulted in a white mold however, and no evidence of the streptomyces. Attempting to grow the bacteria on thicker wood samples, sterilized intact in the autoclave, also resulted in heavy contamination. In one instance, the resulting wood block smelled so vile that I had to discard the piece, in another instance a white mycelium like texture resulted and another resulted in a fuzzy brown-grey contamination with 2 other species on top of it. Herein lies some of the irony of doing this work in a BL1 lab rather than simply burying my samples in the soil outside; these were wild strain bacteria and I was asking them to do what they naturally do on a dead tree stump, decaying leaves, or rotting fruit. But the highly artificial conditions of the lab environment, demanding sterilization and growing predominantly mono-cultures which are then sensitive to contamination, seemed to hinder rather than enable the growth of this bacteria. What makes this interesting though is that other species were able to flourish instead.
[caption id="attachment_3883" align="aligncenter" width="2560"] Image 20: Different Steptomyces griseoviridis cultures in various patterns, as formed by spatially patterning antibiotics, on different species of wood. Photo by Ally Schmaling.[/caption]
Image 20: Different Steptomyces griseoviridis cultures in various patterns, as formed by spatially patterning antibiotics, on different species of wood. Photo by Ally Schmaling.[/caption]
As someone who wants to design the decay of these artifacts in a specific way, I have to reflect on if these accidents do the job just as well and what my priorities are for these artifacts as they change – are they aesthetic? Sensorial? Or programmatic? These factors all have to be weighed, highlighting how bio- designers, as well as scientists, have to decide when it is necessary to guide biology with a firm hand and when it is better to let go. As a classically trained architect, I am used to prescribing not only the aesthetic, but also the performance and sequence of assembly of the things I build, sometimes to a sub-millimeter level. It is very difficult for me to relinquish control of the object to these natural partners. But I have to remind myself that all fabrication tools, including industrial ones, produce results that are always an approximation of the original design, and that it is out of dialogue about what resolution is acceptable that produces some of the most interesting results. While enzymatic degradation offered the most traditional and potentially precise control of where and when degradation might occur, it felt the most mechanistic and least symbiotic of the three methods I tested. This is because the enzymes, while derived from some of the species I was working with, are being used in artificial isolation, separate from the living organism and thus without vitality or agency. Working with the bacteria provided a lot of traditional design space on how to mediate colour and growth patterns as well as introducing their smell as a design element. They are, however, much more challenging to work with compared to the enzymes and the evidence of decay was almost non-existent in the samples. The co-cultures of fungi provided the most effective means of decay as the fungi were robust enough to withstand threats of contamination and grow on the different media more easily. Their appearance is loaded however as they are traditionally viewed as something to be avoided because their presence indicates death and rot. Future work for this project would be to try the same tests outside in a variety of different in situ conditions and comparing how each situation guides how and when decay might take place. Biology is often a black box and for my purposes it is not always necessary to know exactly what is going on at every level so much as it is important to know that under this condition, that will happen. It is very easy to forget this when immersed in the reductive lens of lab work, where in trying to control all variables, we often lose influence over the big picture goal.

[caption id="attachment_3876" align="aligncenter" width="1023"]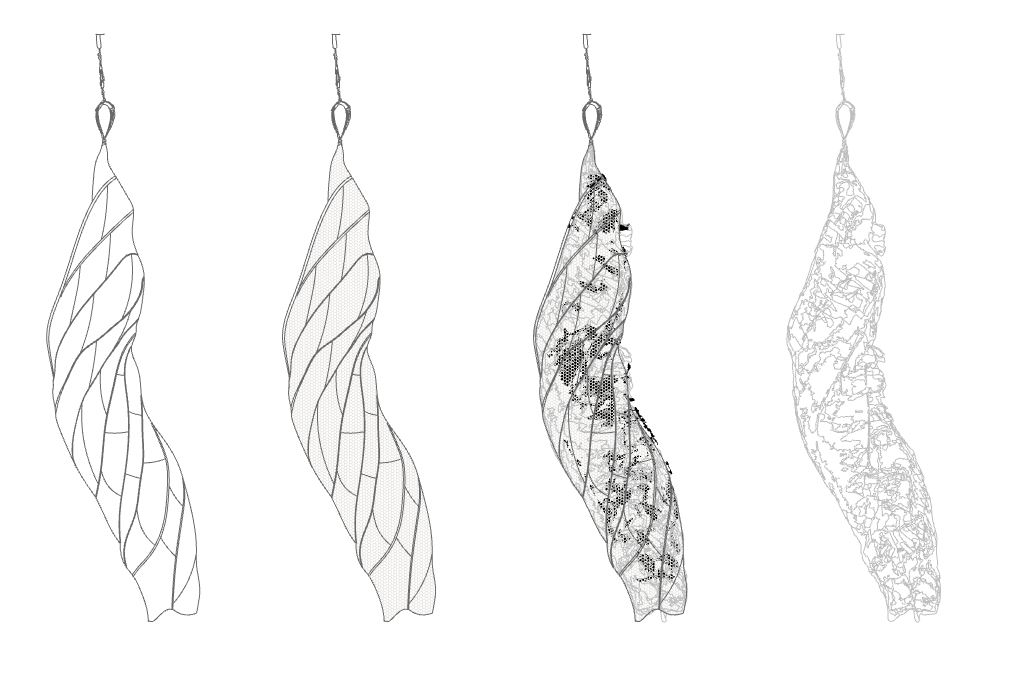 Images 21 & 22: Cocoon for bio-transformation through bacterial colonization made of laser cut chitosan-cellulose composite, with drawing diagramming potential transformation stages. Photos by Ally Schmaling.[/caption]
Images 21 & 22: Cocoon for bio-transformation through bacterial colonization made of laser cut chitosan-cellulose composite, with drawing diagramming potential transformation stages. Photos by Ally Schmaling.[/caption]
[caption id="attachment_3875" align="aligncenter" width="1707"] Image 23: Test of laser cut chitosan-cellulose strips inoculated with Streptomyces coelicolor which failed to grow and heavily contaminated with unknown mold species. Photo by Ally Schmaling.[/caption]
Image 23: Test of laser cut chitosan-cellulose strips inoculated with Streptomyces coelicolor which failed to grow and heavily contaminated with unknown mold species. Photo by Ally Schmaling.[/caption]
In her book, The Value of Everything: Making and Taking in the Global Economy, economist Mariana Mazzucato argues that in our current global economy, value has been misplaced on systems that extract commodity out of everything from oil to the female body, and needs to be reformed to reward systems that create worth instead. Biology is a value creator, the ultimate upcycler, as it uses death and garbage as fuel for new life. It offers more than extracted commodities because it can provide sustained renewal yet it is often valued less because it is inconvenient, challenging, and possesses formal qualities that are not yet standardized or completely predictable. It needs maintenance. But there is so much possibility if we can accept these hurdles.
[caption id="attachment_3912" align="aligncenter" width="1023"] Image 24: Gwion Gwion rock art colonized by symbiotic communities of black fungus and red cyanobacteria, Kimberly, Australia. https://en.wikipedia.org/wiki/Bradshaw_rock_paintings[/caption]
Image 24: Gwion Gwion rock art colonized by symbiotic communities of black fungus and red cyanobacteria, Kimberly, Australia. https://en.wikipedia.org/wiki/Bradshaw_rock_paintings[/caption]
During my interview for this residency, I showed an image of a Gwion Gwion rock painting from Western Australia. Found in locations of extreme sun and rain, the paintings are estimated to be between 20000 to 70000 years old. They remain vibrant because although the initial organic paints to create the images have long since disappeared, what creates the images today are symbiotic colonies of red cyanobacteria and black fungi whose ancestors decomposed and fed on the initial pigments and then flourished within the confines of the original drawing for tens of thousands of years. The paintings exemplify some of the remarkable advantages living material systems have over the non-living – adaptation, repair, and replication, such that out of literal rotting comes a longevity and legacy not possible with man- made artifacts. A handful of the paintings however have overgrown and have lost their original lines. Incorporating biological matter and living organisms in design and manufacture means one is introducing inherent agency into a material system and thus also embracing a degree of risk and uncertainty concerning the final outcome. With this risk and uncertainty, however, is also the potential to incorporate legacy, diversity, and variation during the process which in contemporary industrial practice are either impossible or undesired.
My goal in using this material system for this residency was not to make a bunch of artifacts out of shrimp shells and jam that are easy to dissolve or even to create a zero-carbon footprint project. Rather it was to support an alternative mode of design practice where the process of making and breaking things is not only consumptive but also provisional and to redistribute value away from permanent objects onto transient ones. Ginkgo, by virtue of being a company that designs life, understands how in synthetic biology, value comes from working in symbiosis with the underlying logic of natural systems rather than trying to subjugate them. My question during this residency cannot be “what can I make biology do to help me” (despite my natural controlling tendencies) but rather “how can I bias it such that it benefits the system we exist in, which includes both me and it?” The struggle in a design practice such as mine is to learn to accept the tensions embedded in this mode of practice, where material and biological agency sometimes work in contradiction to what we planned or what we are comfortable with. It is a struggle for industry to accept this inconvenience as well. And so I have to give sincerest thanks to Ginkgo and especially its residency team (Christina Agapakis, Natsai Audrey Chieza, Joshua Dunn, Grace Chuang, and Kit McDonnell), as well as its founders (Tom Knight, Austin Che, Barry Canton, Jason Kelly, and Reshma Shetty) to hosting this residency and supporting more critical reflections on where synthetic biology and bio-design can and may go. It was a great joy and privilege to be able to work at Ginkgo this summer. Thank you!
[caption id="attachment_3845" align="aligncenter" width="2560"] Image 25: Thank you! Photo by Ally Schmaling.[/caption]
Image 25: Thank you! Photo by Ally Schmaling.[/caption]
Posted by Andrea Ling