Ginkgo.Your Innovation Partner.
When a leading pharmaceutical partner hit a roadblock in developing enzymatic solutions for their production process, Ginkgo Bioworks leveraged protein discovery and engineering to develop bio-based manufacturing.
Ginkgo’s engineers developed an end-to-end work package starting from high-throughput assay development through advanced protein engineering, identifying and improving on a new class of enzymes to catalyze an unprecedented activity. Our protein engineering work helped our partner reshape their production process, offering sustainable, cost-effective alternatives to traditional synthetic methods.
Ginkgo Bioworks partnered with a pharmaceutical company to replace traditional synthetic chemistry for a hydroxylation reaction with biocatalysis in drug production, aiming for a lower-cost and more sustainable process. Ginkgo developed an end-to-end solution to develop a novel enzyme for this process.
The work began with custom high-throughput Assay Development, which facilitated precise characterization of multiple phenotypes in large enzyme libraries.
We then identified a strong starting sequence capable of performing the desired reaction. This leveraged Ginkgo’s Ginkgo’s metagenomic sequence database and computational modeling and machine learning tools to select variants for high-throughput screening.
Finally, we optimized the starting sequence towards commercial performance. Zero-shot ML-guided design tools combining sequence- and structure-based modeling enable the design of initial libraries exploring a wide parameter space. Subsequent libraries continue to use these techniques, now augmented by the data from all previous rounds, to recombine observed mutations and identify new ones. This led to order-of-magnitude improvements in each of two difficult phenotypes in only three rounds of engineering.
Ginkgo Bioworks’ Cell Engineering Platform leveraged its proprietary database and AI-enabled tool to discover and engineer biocatalysts for activities that were previously unattainable. Ginkgo’s Enzyme Intelligence suite of tools, paired with a highly-automated Foundry offers our partners the tools they need to identify and leverage the tools they need to have a competitive advantage in the market.
Traditionally reliant on synthetic chemistry for a hydroxylation reaction necessary to produce their small molecule drug, our partner in the pharmaceutical space recognized the potential for a more sustainable and precise approach through biocatalysis. Biocatalysis had the potential to perform this reaction, resulting in enhanced efficiency, reduced environmental impact, and lower cost of production of their target molecule.
However there were no known enzymes that could catalyze the reaction they were achieving through synthetic chemistry. Developing a bio-based solution for this reaction would require them to source, produce, test, and engineer large libraries of enzymes–a research and development project that their team did not have the resources or expertise to execute.
They turned to Ginkgo’s cell engineering platform to help them navigate a large protein discovery and engineering space and develop an enzyme with an activity that had never been described in the literature.
The Assay Development team initiated this collaboration, finding ways to onboard their assay methods for high-throughput screening.
De-novo Assay Design: Ginkgo’s engineers developed a two-pronged assay testing for products of our partner’s reaction by combining EchoMS for rapid, high-throughput primary screening and Chiral HPLC for detailed, precise secondary analysis. This approach ensured comprehensive detection and quantification of reaction products at a scale that allowed screening of large enzyme libraries.
Unexpected Product Discovery: Our assay development uncovered an additional regioisomer that our partner hadn’t anticipated.
Assay Optimization: We optimized the assay to differentiate between our target product and newly discovered byproducts; we adjusted our methods to achieve clear separation and accurate quantification of all relevant chemical species.
After developing a high-throughput assay for the products of interest, we initiated a Metagenomic Enzyme Discovery workflow.
Sequence sourcing: An initial scan of Ginkgo’s metagenomic sequence database using three different seed sequences identified over 183,000 enzyme sequences that had high potential for performing the activity our customer was searching for.
Computational modeling: To narrow down this collection of sequences, we applied molecular dynamics to model substrate docking to identify residue positions associated with substrate binding, catalysis, and dynamical regions implicated in specificity.
Machine learning in metagenomics: Multiple-sequence alignment (MSA) and Language Learning Models were leveraged to score candidate sequences. Statistical sampling and clustering algorithms tuned the diversity of sequences that Ginkgo would test.
High throughput screening: The initial metagenomic library identified 1,000 enzymes that were capable of performing the reaction. Ginkgo’s Foundry expressed these candidates in bacteria and, using the tools developed in the Assay Development phase of this work, identified two sequences with high function. For the first time, we demonstrated that our partner’s reaction could be catalyzed by enzymes; our search found enzymes that resulted in the 0.4mM target product–a key starting point for protein engineering.
Although Protein Engineering improved these enzymes further, optimizing both productivity and selectivity to produce the products of interest, while also minimizing the off-target regioisomers. Each round of protein engineering iterated through well-trod workflows at Ginkgo that led to ultrafast data acquisition: entire cycles of protein design, synthesis, strain construction, testing and data analysis were completed in 7 weeks, allowing our team to make fast, effective decisions in protein engineering.
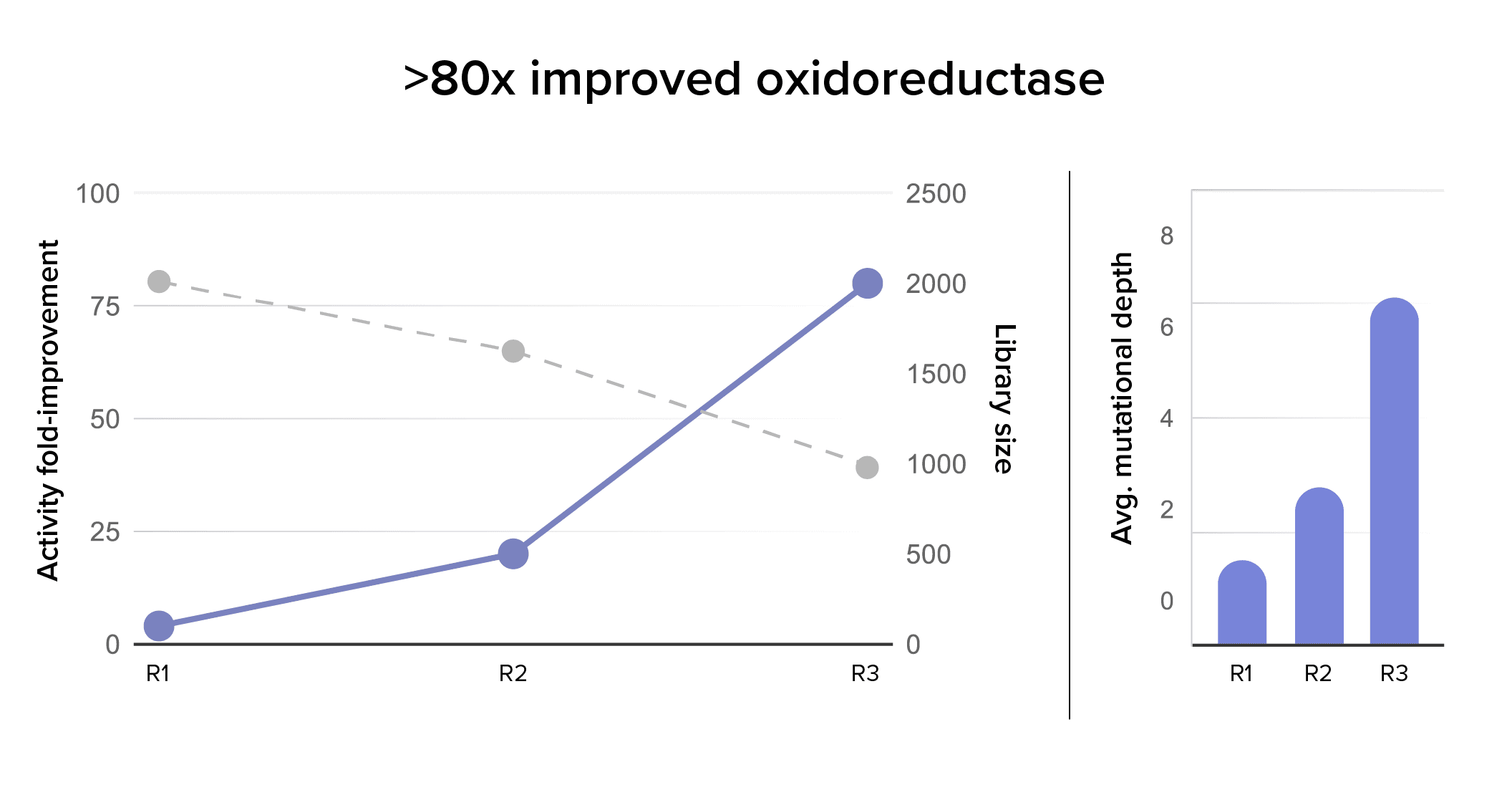
Generation 1: Our first round of protein engineering utilized both sequence (MSA- and LLM-based methods) and structural methods (Molecular dynamics and AlphaFold, among others) to identify improved variants. Result: These results identified variants with >4x improvements in productivity and >10x improvement in selectivity, although typically not together in a single variant. Collectively, all the variants in the generation 1 library were used to train models that were applied to develop the generation 2 library, optimizing for both activity and selectivity.
Generation 2: With our learnings from Generation 1 and our metagenomic campaigns, we leveraged our proprietary OWL technology to engineer enzymes with improved activities.. Our previous campaigns developed a rich data set informing how enzyme sequence affected productivity and selectivity. OWL leveraged this data set to predict which enzymes would have better function. Result: We engineered enzyme sequences with a ~20x improvement in productivity and a 2.5x improvement in selectivity over the starting point.
Generation 3: A final round of enzyme improvement using our machine learning tools resulted in an 80-fold improvement in productivity, and an enzyme that was 17-fold more selective in the reaction products. Result: Ginkgo’s Enzyme Intelligence suite of tools identified an enzyme with an activity never before reported in the literature and optimized it to produce 33mM product, enabling our partner to move from costly and toxic chemical reactions to greener bio-based drug solutions.
Validated Novel Solution: Ginkgo’s work developing a novel biocatalyst to replace the synthetic chemistry in their production process is a first-of-a-kind technology that fundamentally changes their drug production.
Superior Performance Over Traditional Chemistry: Our discovery of an enzyme that performed the reaction in our partner’s process eliminated a step in their original process using synthetic chemistry. Our ability to find this novel enzymatic activity surprised our customer and unlocked a new production process with significant cost savings.
Expanded Project Scope: Our initial success in providing our partner data about the feasibility of a bio-based reaction has led them to extend their collaboration with Ginkgo, aiming to optimize the enzyme’s performance towards commercial viability and to develop a robust manufacturing strain and process.
Broader Application and Strategic Development: Encouraged by these results, our partner is now engaged in identifying and developing new biocatalysts to address other small molecule production challenges in the pharmaceutical field, leveraging the full potential of Ginkgo’s Cell Engineering Platform.
High-throughput assay development: We develop custom assays, targeted to the exact analytes of interest, eliminating the need to rely on proxy or indirect assays. This allows us to collect high-quality characterization data for our enzyme panels and engineer the enzyme for the exact activity needed for commercial success.
Metagenomic Discovery: Ginkgo’s proprietary database is a rich source of diversity for screening new enzymes and protein sequences. Our metagenomic sequence database, coupled with ML tools to search it, can uncover activities never-before described in biology or chemistry.
Fast closed-loop engineering fed by lab data: Our suite of machine learning-enabled protein engineering tools can provide data in 7 weeks. This rapid iterative cycle of design-build-test-learn on Ginkgo’s Cell Engineering Platform can achieve massive improvements, fast.
Ginkgo Bioworks’ Enzyme Intelligence suite of tools enables our partners to achieve their production goals through biology. turns significant technical challenges into opportunities. We work with our partners to optimize pathways to produce their target small molecules production processes. We encourage potential partners to discover how our tools and expertise can elevate your projects, lower costs, and enhance sustainability. Shape the future of biotechnology and drug development with us—contact us to begin exploring revolutionary solutions.